Results and Discussions
⁎ Characterization of the Nanostructures and Immobilized Lipases
⭒ Fourier Transform Infrared Spectroscopy Analysis
FTIR was utilized to analyze the efficiency of covalent immobilization. Figure 1a. shows the FTIR spectra of pure GO and enzyme immobilized GO. The spectrum of GO shows the broadband at 3410 cm-1 that assigned to O-H stretching vibrations of the C-OH groups, the band at 1737 cm-1 shows C=O stretching vibrations of the carbonyl and carboxylic groups and the absorption band at 1620 cm-1 because of the existence of C=C or demonstrates the skeletal vibrations due to unoxidized graphitic domains [45, 46]. The spectra of Cal-B immobilized GO clearly indicate the following bands: 3425 cm-1 (O-H, NH2) due to water and Cal-B, 1647 cm-1 (C=O) corresponding to Amid I, 1550 cm-1 corresponding to Amid II bands of C-N stretching vibrations and N-H bending. Figure 1b. shows FTIR spectra of pure Fe3O4 and lipase immobilized Fe3O4. It can be seen that before lipase immobilization onto Fe3O4, the Fe-O vibrations of Fe3O4 centered at 695 cm-1, after Cal-B immobilization this peak shifted to 650 cm-1 due to the interaction of enzyme and Fe3O4 nanoparticles. The large and broad band is detected at 3423 cm-1 demonstrating the existence of both the OH and NH2 groups of water and Cal-B molecules [32]. Amide I band is the most intense absorption band of proteins that gives a peak at 1643 cm-1. It is caused by the stretching vibrations of the C-N (from 10% to 20%) and C=O groups (from 70 to 85%) [47]. Amid II band is attributed to the N-H bending and C-N stretching vibrations at 1550 cm-1. The presence of several characteristic peaks of GO/Fe3O4 such as Fe-O vibrations (573 cm-1), C–OH stretching vibration (1217 cm-1) [48], aromatic C=C stretching (1600 cm-1) and oxygen stretching vibrations (3440 cm-1) show the successful preparation of GO/Fe3 O4 nanocomposite (Fig. 1c). After enzyme immobilized onto the support, the bands corresponded to chemical structure of enzyme was appeared at 1061 cm-1 (C-N stretching vibrations for aliphatic amines), 1550 cm-1 (Amid II), 1647 cm-1 (Amid I band), 2353–2357 cm-1 (the adsorption doublet band of CO2), and 3440 cm-1 (O-H and N-H absorption). The spectroscopic examination verifies that the enzyme Cal-B was successfully immobilized on the surface of supports.
⭒ Scanning Electron Microscopy Analysis
Figure 2 shows the SEM images of the supports before and after enzyme immobilization. The smooth and regular surface of initial GO/Fe3 O4 and Fe3 O4support can be seen in Fig. 2a, c respectively. After lipase immobilized, the irregular surface of the supports can be visualized in Fig. 2b, d shows that the supports are filled unevenly by the lipase.
Figure 1-a, b, c
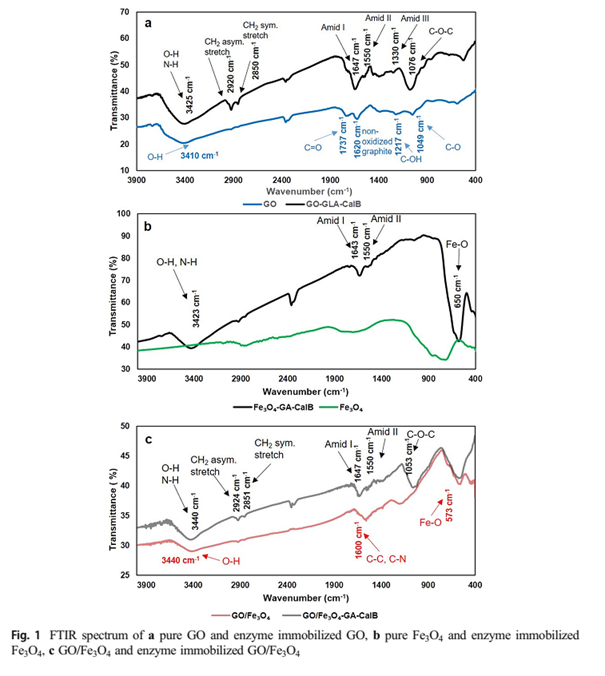
Figure 2-a, b, c, d

⭒ The Effect of pH and Temperature on Immobilization
One of the most important parameters that cause alteration of the enzyme activity in aqueous solutions is pH. The pH change of the medium during immobilization affects the tertiary structure and active site of the enzyme molecules [49]. To determine the impact of pH on immobilization, activation of the supports (with crosslinkers, 30 °C, 4 h) and the immobilization of Cal-B onto the supports (30 °C, 12 h) were carried out at various pH values (pH 5–8). The effect of pH on the immobilization protocol is shown in Fig. 3a. The highest enzyme activity was obtained at pH 6 (13.29 U/mg) for GO-GLA-CalB enzyme, at pH 7 (11.85 U/mg) for Fe3O4-GLA-CalB and at pH 8 (12.96 U/mg) for GO/Fe3O4-GLA-CalB. When these values are compared with the free enzyme activity (18.52 U/mg, pH 6, 37 °C) in the optimum conditions, it can be seen that the activities of three immobilized enzymes decreased. The relative activities of immobilized samples are 72%, 64%, and 70% for GO-GLA-CalB, Fe3O4-GLA-CalB, and GO/Fe3O4-GLA-CalB, respectively. The pH values demonstrate that there is no significant conformational change in the GO-GLA-CalB (pH 6) and Fe3O4-GLA-CalB (pH 7) enzymes during covalent binding
Figure 3-a, b
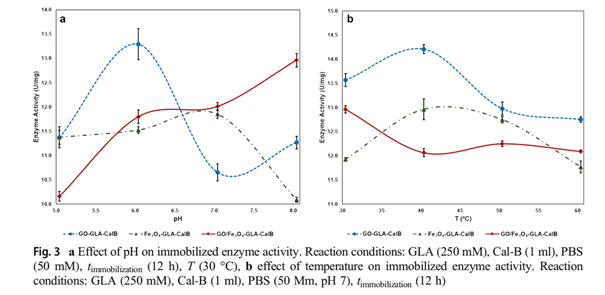
However, the results represent that lipase immobilized onto GO/Fe3O4-GLA-CalB exhibits higher resistance to pH (pH 8.0). Miletic et al. [50] found similar result in their study for Cal-B immobilized on polystyrene nanoparticles. In order to observe the effect of reaction temperature on immobilization, the activation of the supports and the immobilization protocol were carried out at different temperatures (30–60 °C, pH 7.0), and the results are shown in Fig. 3b. The optimum reaction temperature of the free lipase is 30 °C, while the immobilized enzymes represent the best catalytic activities at 40 °C. At this temperature, the relative activities of GO-GLA-CalB, Fe3O4-GLA-CalB, and GO/Fe3O4-GLA-CalB are 74.2%, 81.4%, and 75.15%, respectively. Activity recovery of Candida rugosa enzyme immobilized onto GO/Fe3O4 nanocomposite was obtained as 64.9% by Xie and Huang [37]. According to these results, their resistance to thermal denaturation is better than the free lipase. As the result of the immobilization, molecules have limited conformational mobility, thus stability is increased against various deactivating forces. Similar values were obtained by Y ılmaz et al. [51] when the Candida rugosa lipase (CRL) was encapsulated within chemically inert sol-gel composite matrixes. The main reason of the improved stabilitiy is the covalent bonds between the enzyme and the matrix, which restrict the conformational mobility of the molecules and cause the rigidity and stability of the enzyme to increase [52]. The reaction time is very important for enzyme immobilization. If there is an insufficient interaction, the enzyme cannot bind on the support or the enzyme may be denatured because of a longer duration of the interaction. Figure 4 shows the catalytic activity of immobilized lipases prepared at various immobilization times. As seen in Fig. 4., it is clear that the catalytic activities of the three immobilized enzymes are different for all immobilization times. Fe3O4-GLA-CalB shows the best activity for 16 h. At this time, the catalytic activity of Fe3O4-GLA-CalB is 13.73 U/mg. However, the catalytic activities decreased after 16 h. This phenomenon can be related to denaturation of lipases at a long time. Optimum immobilization time for GO-GLA-CalB and GO/Fe3O4-GLA-CalB is 12 h at which the activities are 14.17 U/mg and 13.12 U/mg, respectively. Longer time slightly reduced the immobilization yield on the support. It could be concluded that the
Figure 4
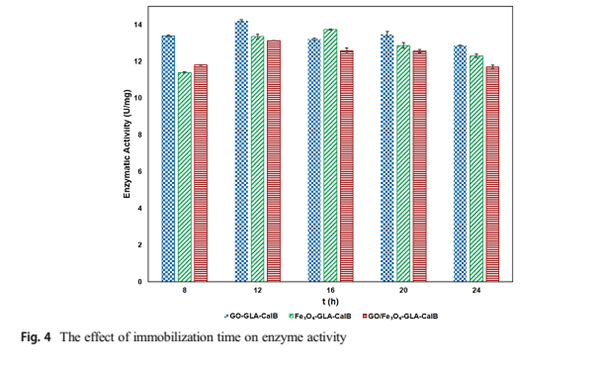
immobilized enzymes have different structures and show different activities at different immobilization times.
⁎ The Effect of Crosslinkers Concentration on the Catalytic Activity
Enzyme immobilization by covalent binding generally includes two steps. First, the support surface is activated using bifunctional agents, and then the enzyme is immobilized on the activated surface covalently. Crosslinkers, often used in covalent binding, behave as a bridge between the support material and enzyme molecules [53]. In this part of the study, activation of the supports was carried out with varying quantities of GLA and EPH (150, 200, 250, and 300 mM) for 4 h at 30 °C. Then, the free enzyme was immobilized onto these activated supports as previously mentioned. When GO was activated by glutaraldehyde, with the increasing of glutaraldehyde concentration the activity of immobilized enzyme increased, after 250 mM (2.36%, v/v) of glutaraldehyde concentration the activity decreased. When Fe3O4 was activated with glutaraldehyde, the highest immobilized enzyme activity was obtained when 300 mM (2.83%, v/v) of glutaraldehyde was used. For GO/Fe3O4 support material, 200 mM (1.90%, v/v) of glutaraldehyde represented the highest enzyme activity, after 200 mM (1.90%, v/v) of glutaraldehyde the activity decreased. The three immobilized lipases gain different conformations from each other after the immobilization protocol; therefore, their highest activities are detected at different glutaraldehyde concentrations. However, for each sample, with the increasing GLA concentration, the enzyme activity increased because of the increased concentration of aldehyde groups on the support surfaces. On the other hand, after a certain GLA concentration, the activities of the immobilized preparations were decreased, at a high GLA concentration, the multiple chemical bonds might be generated between the support material and the enzyme molecules [54]. When the carriers were activated with epichlorohydrin at different concentrations, it was observed that enzyme activities were lower than glutaraldehyde (Table 1). The results prove glutaraldehyde is more effective than epichlorohydrin on the enzymatic activity.
⁎ The Effect of Surfactants on Enzyme Activity
The existence of surfactants during immobilization increases the thermal and chemical stability of enzymes [55, 56]. The effect of increasing concentration of non-ionic surfactants Triton X- 100 and Tween 80 were analyzed in this study, and the results were presented in Table 2. In the same conditions, without the use of surfactants, the activity of enzyme immobilized on GO, Fe3O4 and GO/Fe3O4 were 15.33, 14.26, and 13.47 U/mg, respectively (Table 1). When 30 mM (3.7% v/v) and 40 mM (4.9%, v/v) of Tween 80 were used, the highest immobilized enzyme activities were 15.03, 14.72, and 13.56 U/mg for GO-GLA-CalB, Fe3O4-GLA-CalB, and GO/Fe3O4-GLA-CalB, respectively. The higher concentration of the surfactants caused the decrease in the activity of all three immobilized enzymes. The similar behavior was also observed when Triton X-100 was used as the surfactant. However, the increase in concentration for both surfactants did not have a significant effect on immobilized enzyme activities. There are two possible considerations to explain why there is no significant change in enzyme activity: (i) The interaction time (45 min) between the surfactants, and the support materials is insufficient. (ii) The use of surfactants during the activation process of the support material rather than the enzyme immobilization process.
⁎ Enantioselective Transesterif ication of 1-Phenyl Ethanol with Immobilized Lipases
In this study, Cal-B immobilized onto three nanostructures, and the immobilized lipases, Fe3O4-GLA-CalB, GO-GLA-CalB, and GO/Fe3O4-GLA-CalB, catalyzed the transesterification of 1-phenyl ethanol with vinyl acetate when used in hexane with the activity of 15.33 U/mg, 14.26 U/mg, and 13.47 U/mg, respectively (Table 1). Enantioselective transesterification of 1-phenylethanol assayed for three immobilized enzymes for different reaction times. As shown in Fig. 5a, b, and c enantioselectivity is considerably influenced by the different immobilized enzymes. Fe3O4-GLA-CalB showed poor enantioselectivity (ee% = 23.00, E = 3.77) with 31.36% conversion in 15 h. Significant changes observed when lipases GO-GLA-CalB and GO/Fe3O4-GLA-CalB catalyzed the reaction. The highest values of enantioselectivity (E = 507.74, 375.77), enantiomeric excess (ee > 99%, 94.32%), and conversion (c = 50.73%, 49.02%) were obtained when GO-GLA-CalB and GO/Fe3O4-GLA-CalB catalyzed the reaction, respectively. This is probably due to the fact that enzymes with different conformations and physical properties that obtained by the immobilization of Cal-B onto
Table 1 The effect of glutaraldehyde and epichlorohydrin concentrations on the activity of the immobilized enzyme
| Immobilized enzyme activity, U/mg | GO-CalB | Fe3O4-CalB | GO/Fe3O4-CalB |
|---|---|---|---|
| Cross linker concentration, mM | GLA EPH | GLA EPH | GLA EPH |
| 150 | 13.47 12.28 | 13.46 11.81 | 13.03 11.70 |
| 200 | 14.45 13.44 | 13.83 12.98 | 13.47 12.41 |
| 250 | 15.33 13.59 | 14.26 12.46 | 13.12 12.53 |
| 300 | 15.24 14.22 | 14.45 13.16 | 11.85 11.69 |
Table 2 The effect of glutaraldehyde and epichlorohydrin concentrations on the activity of the immobilized enzyme
| Immobilized enzyme activity, U/mg | GO-CalB | Fe3O4-CalB | GO/Fe3O4-CalB |
|---|---|---|---|
| Surfactant concentration, mM | Tween 80 Triton X-100 | Tween 80 Triton X-100 | Tween 80 Triton X-100 |
| 10 | 14.45 13.99 | 13.92 13.83 | 13.03 13.29 |
| 20 | 14.57 14.00 | 14.35 13.52 | 13.33 13.47 |
| 30 | 15.03 14.57 | 14.72 14.04 | 13.46 13.53 |
| 40 | 14.58 14.63 | 14.34 14.35 | 13.56 13.70 |
| 50 | 14.04 13.44 | 14.04 14.23 | 12.71 13.34 |
different support materials. Since the enzyme is attached directly to the support material, the supports having different morphological and physical properties affect the catalytic properties of the enzyme [26, 57]. Thus, the support material used in the immobilization affects the biological activity of the enzyme by altering the properties of the enzyme [58].
⁎ Operational Stability
When the best immobilization conditions, support material, and immobilization method are selected, the activity and the stability of the immobilized enzyme relative to the free enzyme
Figure 5-a, b, c
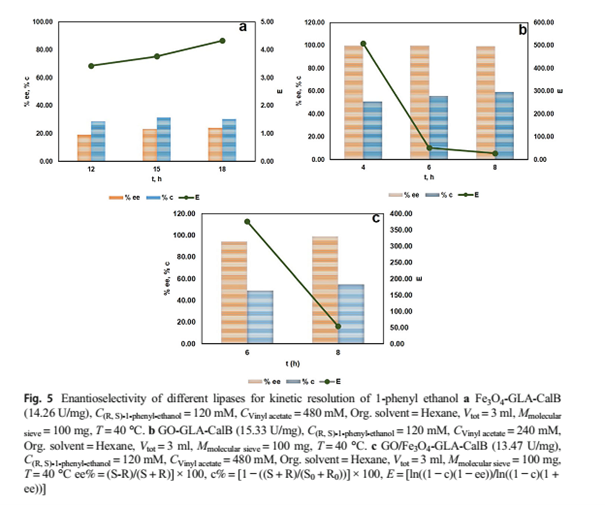
Figure 6
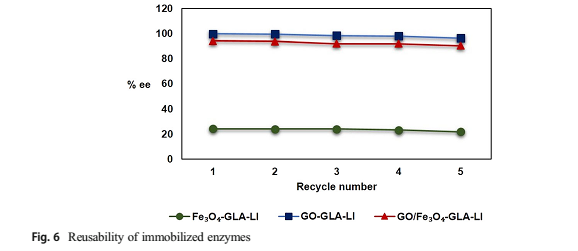
may increase. Recovery and repeated reusability of immobilized enzymes in catalytic systems are another important parameters. To determine the reuse of immobilized enzymes subsequent cycles for three immobilized enzymes were carried out separately. Each of them was used for different reaction conditions, but each cycle was carried out on the same conditions of reactions. After the first and further reaction cycles of the kinetic resolution of 1- phenylethanol, the immobilized lipases were regained from the reaction medium with the use of a magnet or via filtration and washed 3 times with hexane. Then, they were dried to remove the organic solvent, and they were used in the new reaction system. The experiment was repeated up to five reaction cycles with the same immobilized lipases. Figure 6 shows the reusability of immobilized enzymes on Fe3O4, GO and GO/Fe3O4. It is seen that there is no significant decrease in enantiomeric ratios and this enzymatic process can be repeated five times without important loss of enantioselectivity.